Exploring The Different Types Of Combustion Reactions

When you hear the word "combustion," what comes to mind? Fire, explosions, maybe even a little bit of danger? Well, it’s time to put on your science hat because we’re diving into the fascinating world of combustion reactions. Whether you're a student, a curious mind, or someone in the industrial sector, understanding the different types of combustion processes can illuminate not only chemistry but also its practical applications in engines and industry.
What Is Combustion?
Combustion is a chemical reaction between a fuel and an oxidant that produces heat and light. It’s like a fiery dance where energy is released, transforming potential energy into kinetic energy. The fuel can be anything from wood to gasoline, and the oxidant is usually oxygen from the air.
But not all combustion is created equal! There are various types of combustion processes, each with its own characteristics, products, and applications. Let’s break them down.
Types of Combustion in Chemistry
1. Complete Combustion
What is it?
Complete combustion occurs when a fuel burns in sufficient oxygen, producing carbon dioxide (CO₂) and water (H₂O) as the main products. Think of it as the “ideal” combustion scenario.
Key Features:
- Produces maximum energy.
- Minimal pollutants—mostly just CO₂ and H₂O.
- Common in natural gas stoves and furnaces.
Example:
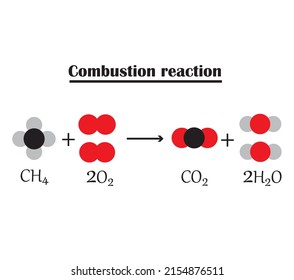
When methane (CH₄) burns completely, the reaction looks like this:
[ CH₄ + 2O₂ \rightarrow CO₂ + 2H₂O + \text{Energy} ]
2. Incomplete Combustion
What is it?
Incomplete combustion occurs when there isn’t enough oxygen for the fuel to react completely. This leads to the production of carbon monoxide (CO), soot, or other hydrocarbons, alongside CO₂ and H₂O.
Key Features:
- Less energy is released.
- Produces harmful pollutants like CO, which is toxic.
- Common in engines and poorly maintained heating systems.
Example:
In the case of incomplete combustion of methane, the reaction might look like:
[ 2CH₄ + 3O₂ \rightarrow 2CO + 4H₂O + \text{Energy} ]
3. Rapid Combustion
What is it?
Rapid combustion happens quickly, releasing energy in the form of heat and light. This is what you typically see in fireworks or a campfire.
Key Features:
- Produces bright flames and a lot of heat.
- Used in engines, explosives, and fireworks.
4. Slow Combustion
What is it?
Slow combustion is a gradual process that releases energy over a long period. Think of a log burning in a fireplace—it doesn’t just go up in flames; it smolders.
Key Features:
- Occurs without flames.
- Useful in processes like respiration in living organisms.
5. Spontaneous Combustion
What is it?
Spontaneous combustion occurs when material ignites without an external flame or spark, usually due to heat buildup from chemical reactions within the material.
Key Features:
- Can be dangerous and unpredictable.
- Commonly seen in compost heaps or oily rags.
6. Explosive Combustion
What is it?
Explosive combustion is an extremely rapid reaction that produces a shockwave. Think dynamite or gasoline in an engine.
Key Features:
- Releases a massive amount of energy in a short time.
- Used in demolition and automotive industries.
Types of Combustion in Engines
Combustion plays a crucial role in various types of engines. Here’s a quick rundown:
-
Internal Combustion Engines: Used in cars, these engines rely on the combustion of fuels like gasoline or diesel. Here, both complete and incomplete combustion can occur, depending on the engine’s efficiency.
-
External Combustion Engines: Examples include steam engines, which use coal or wood to heat water, generating steam that drives a piston.
-
Jet Engines: These engines rely on rapid combustion to propel aircraft. They use kerosene-like fuels that combust quickly and efficiently to produce thrust.
Types of Combustion in Industrial Applications
Combustion isn’t just a chemistry lab phenomenon; it’s a cornerstone of many industrial processes:
-
Power Generation: Fossil fuels like coal, natural gas, and oil are combusted in power plants to generate electricity.
-
Manufacturing: Industries burn fuels to produce heat for processes like metalworking, glass production, and chemical manufacturing.
-
Waste Management: Incineration is a method of burning waste materials to reduce volume and generate energy.
Conclusion
Understanding the different types of combustion reactions not only enhances your knowledge of chemistry but also opens your eyes to their real-world applications. From powering your car to generating electricity, combustion processes are everywhere!
So, next time you see a flame or hear the roar of an engine, remember: there's a whole lot of science at play. Whether it's complete combustion lighting up your evening bonfire or incomplete combustion creating that pesky carbon monoxide in your car, combustion is both fascinating and essential.
Stay curious, and keep exploring the fiery wonders of chemistry! If you have any questions or want to dive deeper into a specific type of combustion, drop a comment below. 🔥
