Exploring the Different Types of Chemical Reactions: A Comprehensive Guide
Chemical reactions are the heart and soul of chemistry, where substances undergo transformations to create something new. Ever wondered how your morning coffee brews or why rust forms on your bike? Spoiler alert: it’s all about chemical reactions! In this guide, we'll dive into the different types of chemical reactions explained, uncover fascinating examples of chemical reaction types in chemistry, and explore the classification of chemical reactions with examples. So, buckle up and prepare to impress your friends with your newfound knowledge of common types of chemical reactions in everyday life!
What Are Chemical Reactions?
At its core, a chemical reaction involves the breaking and forming of bonds between atoms. This process leads to the alteration of the chemical structure of substances. Whether it’s a bubbling potion in a science lab or the slow decay of organic matter, chemical reactions are everywhere!
Why Should You Care?
Understanding chemical reactions not only helps in academics but plays a vital role in fields like environmental science, engineering, and even cooking! Plus, knowing how these reactions work can help you appreciate the world around you.
The Big Picture: Types of Chemical Reactions
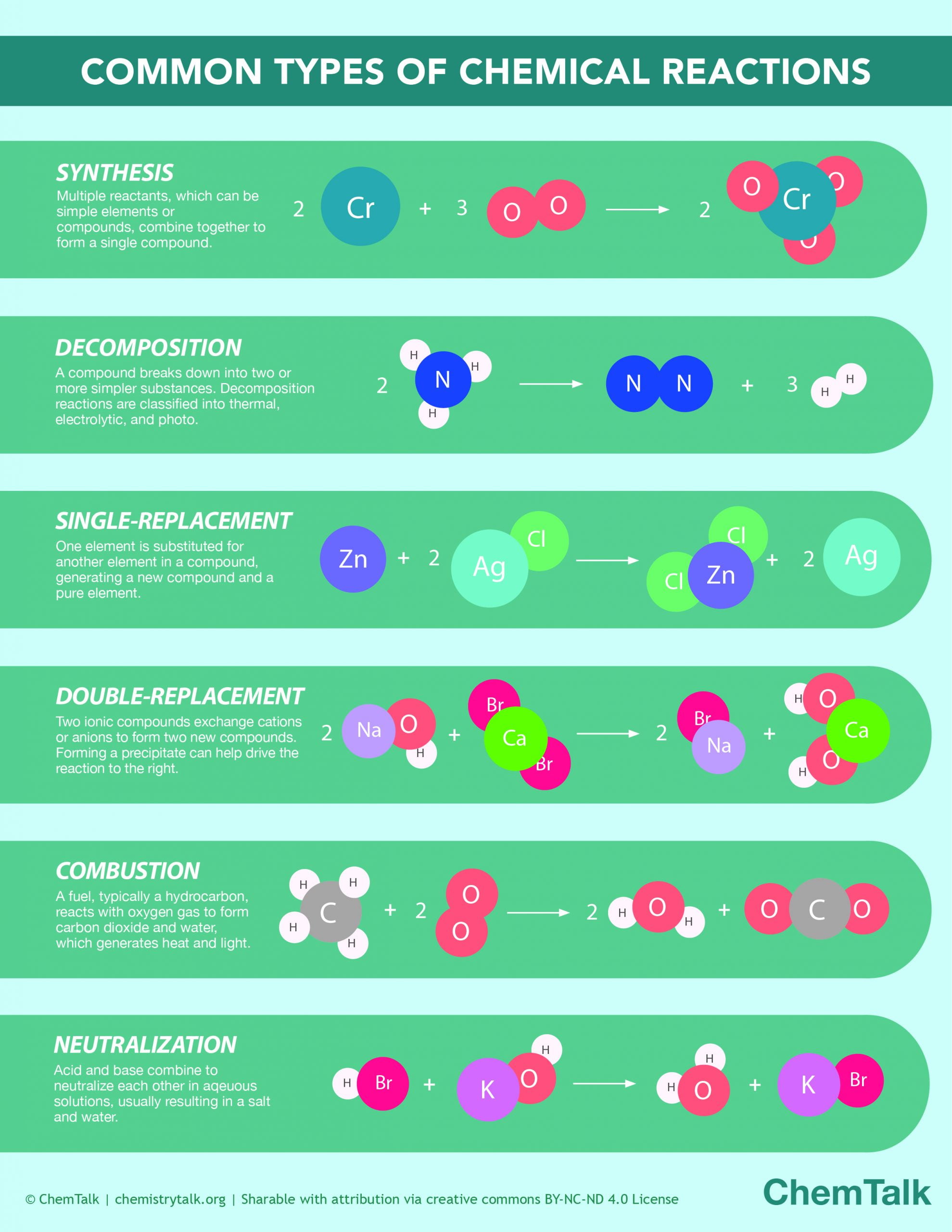
So, what exactly are the types of chemical reactions and their characteristics? In general, chemical reactions can be categorized into five main types. Let’s break them down!
1. Synthesis Reactions
Definition: Two or more reactants combine to form a single product.
Characteristics:
- Involves simple substances creating complex ones.
- Often exothermic (releases heat).
Example:
[ \text{A + B} \rightarrow \text{AB} ]
Think about how hydrogen and oxygen combine to form water!
2. Decomposition Reactions
Definition: A single compound breaks down into two or more simpler substances.
Characteristics:
- Requires energy input (heat, light, or electricity).
- Often endothermic (absorbs heat).
Example:
[ \text{AB} \rightarrow \text{A + B} ]
Consider the electrolysis of water, where water is split into hydrogen and oxygen gas.
3. Single Replacement Reactions

Definition: An element replaces another element in a compound.
Characteristics:
- Involves one reactant being displaced.
- Typically occurs in ionic compounds.
Example:
[ \text{A + BC} \rightarrow \text{AC + B} ]
Imagine zinc displacing copper in a copper sulfate solution.
4. Double Replacement Reactions
Definition: Exchange of ions between two compounds.
Characteristics:
- Often occurs in aqueous solutions.
- Can produce a precipitate or gas.
Example:
[ \text{AB + CD} \rightarrow \text{AD + CB} ]
When mixing silver nitrate and sodium chloride, you get a lovely white precipitate of silver chloride!
5. Combustion Reactions
Definition: A substance combines with oxygen, releasing energy in the form of light or heat.
Characteristics:
- Typically involves hydrocarbons.
- Produces carbon dioxide and water as products.
Example:
[ \text{C}_x\text{H}_y + \text{O}_2 \rightarrow \text{CO}_2 + \text{H}_2\text{O} ]
Think of burning wood or gasoline—classic combustion reactions!
Real-Life Applications: Common Types of Chemical Reactions
You might be wondering, "What are some common types of chemical reactions in everyday life?" Let’s take a closer look!
Cooking
- Baking: Yeast in bread undergoes fermentation, a type of chemical reaction that produces carbon dioxide, causing the dough to rise.
- Caramelization: When sugar is heated, it undergoes a decomposition reaction, transforming into delicious caramel!
Cleaning
- Vinegar and Baking Soda: This classic combo creates a double replacement reaction that produces carbon dioxide gas, perfect for unclogging drains!
Rusting
- Iron + Oxygen + Water: This is a slow oxidation reaction, a type of combustion that leads to the formation of rust.
Digestion
- Food Breakdown: Enzymes in your stomach catalyze decomposition reactions that break down food into simpler molecules for absorption.
Classification of Chemical Reactions with Examples
To wrap things up, let’s summarize the classification of chemical reactions with examples to help solidify your understanding:
| Reaction Type | General Equation | Example |
|---|---|---|
| Synthesis | A + B → AB | 2H₂ + O₂ → 2H₂O |
| Decomposition | AB → A + B | 2H₂O → 2H₂ + O₂ |
| Single Replacement | A + BC → AC + B | Zn + CuSO₄ → ZnSO₄ + Cu |
| Double Replacement | AB + CD → AD + CB | AgNO₃ + NaCl → AgCl + NaNO₃ |
| Combustion | CₓHᵧ + O₂ → CO₂ + H₂O | CH₄ + 2O₂ → CO₂ + 2H₂O |
Conclusion: Your Chemical Reaction Cheat Sheet
Congratulations! You’re now equipped with a solid understanding of the different types of chemical reactions and their significance in both science and daily life. From the fascinating world of synthesis reactions to the explosive excitement of combustion, chemical reactions are a fundamental part of reality.
Next time you enjoy a refreshing soda or watch a campfire crackle, you’ll appreciate the science behind it! Keep exploring, stay curious, and maybe even try a new recipe or experiment at home. Who knows what kind of reactions you’ll spark?
By keeping this guide handy, you’ll not only impress your friends but also enhance your understanding of the world. Happy experimenting!
